Elastomers: alternative to stainless steel in PEM batteries

Zero-emission mobility is more likely to come true every day with rapidly growing hydrogen fuel cells (PEMFC) technology.
As one of the most efficient energy-conversion devices, PEM fuel cells convert oxygen from air and hydrogen into clean energy, leaving water as the only by-product. Their low operating temperature and excellent reliability make them particularly suitable for wide applications as power sources for commercial vehicle market.
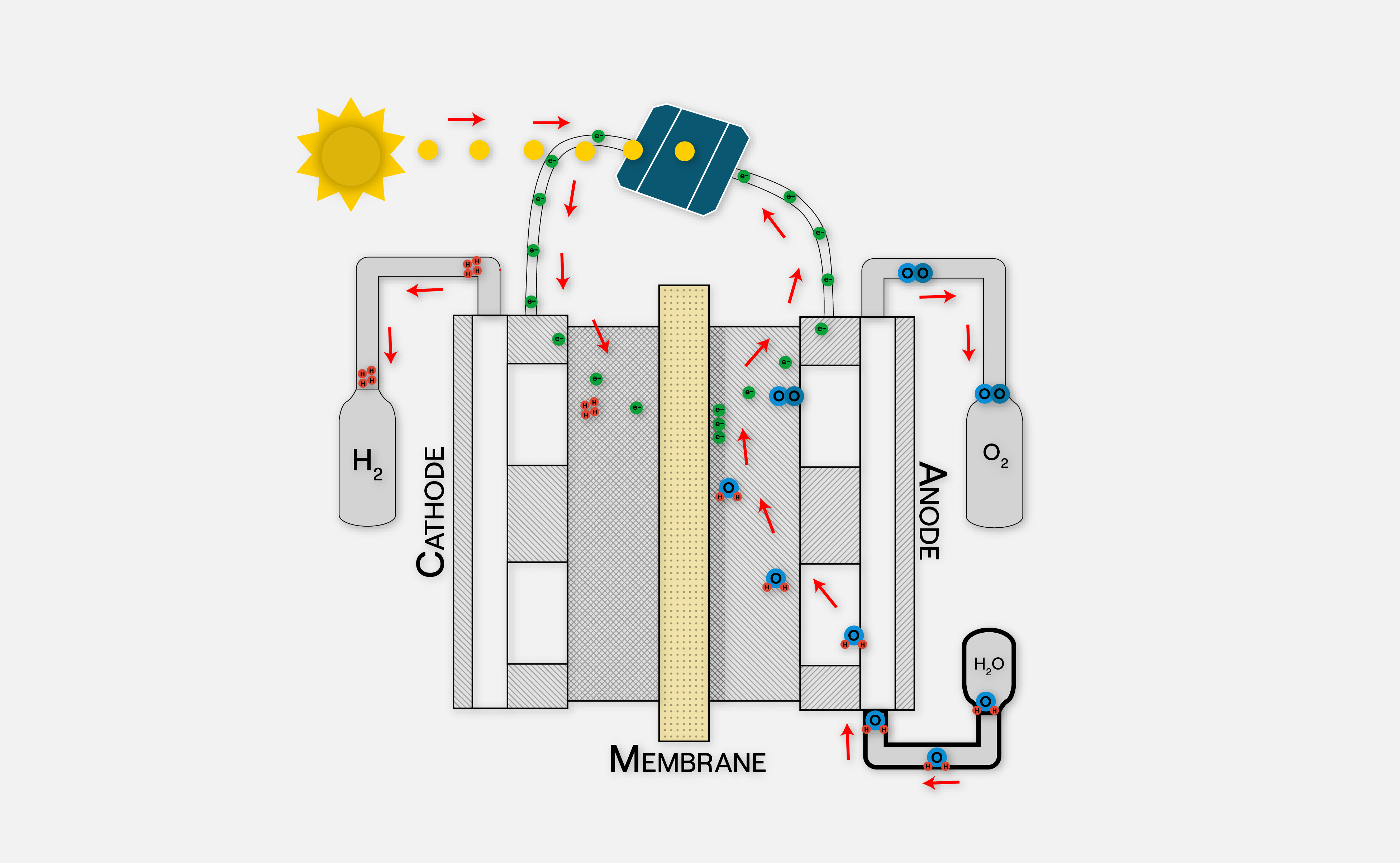
How does a Hydrogen Fuel Cells Work?
Hydrogen fuel cells, parallelly connected in ‘stacks’, generate electricity through a series of chemical reactions that lead to water formation. On the anode, hydrogen gives up its electrons, producing H+ ions. The separated electrons then travel through an external circuit to the cathode as current, which is used to power an electric traction system. In the cathode, the oxygen reacts with the incoming H+ ions and electrons to form hydroxyls OH-, which further react with H+ ions and electrons to produce water.
Even if the working principle seems simple, PEMFC are very delicate systems whose components need to be carefully chosen. The catalyst used at the electrodes is based on costly platinum, making it easy to poison the cell with foreign particles that might come from the fuel or stack components.
Increasing demands on Hydrogen Fuel Cells materials: durability, purity, weight.
Catalyst or membrane poisoning can result in a dramatic performance drop for the fuel cell, shortening its lifespan considerably. The durability of the cell directly influences its cost, hence high demands are placed on the selection of the right materials of the cell components to increase the system's reliability.
Purity problems in metallic materials.
Stainless steel is a suitable material for structural equipment in fuel cells such as piping, gas cylinders or valves. Owing to its toughness and ductility, the use of stainless steel is widely preferred in fuel cell parts such as piping and high-pressure tanks, where contact with high-purity and high-pressure hydrogen occurs.
Despite its many qualities, stainless steel in prolonged contact with hydrogen suffers important structural changes that have a negative impact on the fuel cell system, not to mention its high weight and cost. Some of the main concerns related to the use of metals in PEMFC are phenomena such as the hydrogen embrittlement and corrosion.
Hydrogen embrittlement occurs when hydrogen enters and diffuses into metals. Consequently, these become brittle. This phenomenon is caused by the fact that the hydrogen molecule in contact with metals splits into two atoms and enters the material in an atomic state, causing a harmful effect. The extent of embrittlement depends on the amount of hydrogen absorbed and the microstructure of the material.
Hydrogen embrittlement can happen in all situations where hydrogen is in direct contact with metallic parts, such as hydrogen tank and the anode loop of the fuel cell.
Durability problems in metallic materials.
Another phenomenon threatening the lifespan of metal parts is their susceptibility to corrosion, especially when the metal is not coated. Metals may suffer dissolution in the acidic and humid environment of PEM fuel cells, leaching ions that may poison the membrane electrode assembly, causing a decrease in the power output of the fuel cell.
Elastomers: the solution for hydrogen fuel cells
For hoses and gaskets in fuel cell systems, elastomers are an ideal solution. Their high degree of flexibility and light weight make them perfect candidates for hoses of complicated shapes that must fit under the hood of fuel cells vehicles.
Hydrogen embrittlement does not occur in elastomers, since in polymers, hydrogen is absorbed as a diatomic molecule. The effect of hydrogen on elastomers is based on the permeability, diffusivity, and solubility of hydrogen in them. These parameters vary according to elastomer family and the ingredients chosen. For instance, the permeability of silicone rubber is much higher than the permeability of fluorocarbon-based fluoroelastomers.

The compounding recipe also has an impact on the purity of the material and the ions that might leach from it. For example, the acceptable leaching values of elements such as halogens (F, Cl, Br, I), alkali metals (Li, Na, K, Rb, Cs, Fr) and alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra) must be evaluated.
In Venair, compounds for fuel cell hoses are tailored to meet desired characteristics and control the amount of leaching substances, while low permeability to hydrogen is maintained.
SOURCES
https://www.frontiersin.org/articles/10.3389/fenrg.2014.00002/full
https://www.twi-global.com/technical-knowledge/faqs/what-is-hydrogen-embrittlement
https://www.technology.matthey.com/article/57/4/259-271/